PROSTATE CANCER: HORMONAL THERAPY
Includes 2021 CUA Guidelines on Androgen Deprivation Therapy: Adverse Events and Management Strategies§
Testosterone
- See section in Male Reproductive Physiology Chapter Notes
Androgens and the Prostate
- The primary androgen of the prostate is dihydrotestosterone (DHT)
- Testosterone is converted to DHT by 5α-reductase
- Type 1 5α-reductase is expressed primarily in the non-genital skin and liver, and to a lesser extent in the prostate, testis, and brain
- Type 2 5α-reductase is expressed predominantly in the prostate epithelium and other genital tissues such as the epididymis, genitalia, seminal vesicle, testis, but also in liver, uterus, breast, hair follicles, and placenta
- Functional type 2 5α-reductase is a prerequisite for normal development of the prostate and external genitalia in males
- Males with inherited 5α-reductase deficiency have miniscule prostatic tissue
- DHT binds to intracytoplasmic ARs with much greater affinity than testosterone.
- Binding of DHT to the AR enhances translocation of the steroid-receptor complex into the nucleus and activation of androgen response elements
- Serum testosterone elevations are known to occur with use of a 5-ARI (both finasteride and dutasteride), but values will typically remain within the normal laboratory range§
- Intraprostatic testosterone levels are increased with the use of a 5-ARI§
- Lack of testosterone can protect against development of prostate cancer, however, even hypogonadal men can develop prostate cancer and their cancers may be driven by androgen independent pathways
- The role of the androgen receptor is well established in the progression of castrate-resistant prostate cancer
The Androgen Receptor (AR) and Castrate-Resistant Prostate Cancer (CRPC)
- The AR is a ligand-inducible transcription factor, meaning that they cause transcription of target genes within specific cells after ligands (e.g., testosterone) bind to them
- Molecular mechanisms implicated in the process of castration resistance (5):
- Hypersensitivity: the AR pathway can become hypersensitive through a variety of molecular alterations (such as gene amplification) and be activated by even lower levels of androgen
- Promiscuity: the AR can be activated by ligands other than androgen
- Outlaw: growth factor peptides such as epidermal growth factor and insulin-like growth factor-1 increase AR transcriptional activity in the absence of androgen
- Bypass: activation of parallel or alternative survival pathways allows otherwise androgen-dependent prostate cancer cells to survive in the absence of androgen
- Lurker cell: a small population of possible epithelial stem cells is pre-existent in the prostate and androgen deprivation selects for the outgrowth of these castration-resistant cells.
- ADT should continue in CRPC
- Even when prostate cancer progresses despite castrate levels of androgen i.e. CRPC, it is rarely resistant to androgen action
- Exogenous androgen results in symptomatic tumor flare in 87% of patients with CRPC
- Although the term hormone-refractory prostate cancer has been widely used to describe a state of progressive disease despite ADT, the term castration-resistant prostate cancer is more clinically precise and relevant
- Even when prostate cancer progresses despite castrate levels of androgen i.e. CRPC, it is rarely resistant to androgen action
Mechanisms of androgen axis blockade
- History
- In 1946, Charles Huggins demonstrated the influence of hormonal therapy in prostate cancer. He studied 8 men with metastatic prostate cancer and found that bilateral orchiectomy and estrogen reduced serum acid phosphatase (PSA wasn't discovered until 1979), while testosterone therapy increased serum acid phosphatase.§ He won the nobel prize in 1966 for his work.
- Current therapeutic approaches for androgen axis blockade (4): inhibit source, stimulation, synthesis, and receptor
- Ablation of sources (bilateral orchiectomy)
- Inhibition of stimulation i.e. luteinizing hormone–releasing hormone (LHRH/GnRH) and/or LH release (estrogen, LHRH agonists/antagonists)
- Inhibition of synthesis (ketoconazole, abiraterone)
- Androgen-receptor antagonists i.e anti-androgens (cyproterone acetate, flutamide, bicalutamide, enzalutamide, apalutamide, daralutamide)
- Ablation of androgen sources
- Bilateral orchiectomy
- Quickly reduces circulating testosterone levels to < 50 ng/dL (considered castrate).
- Within 24 hours, testosterone levels are reduced by > 90%
- Has largely been replaced by LHRH analogues
- Subcapsular orchiectomy (removal of glandular tissue only) has been advocated as a technique of ADT that avoids the psychological consequences of an empty scrotum
- Quickly reduces circulating testosterone levels to < 50 ng/dL (considered castrate).
- Inhibition of LHRH and/or LH release
- Estrogen
- Historical
- First agent used at a central inhibitor
- Largely replaced by LHRH analogues
- MOA: potent negative feedback of estrogen on LH secretion
- Estradiol is 1000x more potent at suppressing LH and FSH secretion than testosterone
- Diethylstilbestrol (DES)
- As effective as surgical castration
- Association with cardiovascular toxicity has limited its use
- LHRH agonists (-relin/-rolide, Goserelin (Zoladex), Triptorelin (Trelstar) and Leuprolide (Lupron))
- As effective as orchiectomy
- Initially, the clinical utility of LHRH agonists were hampered by their short half-life, requiring daily injections. The generation of long-acting depot preparations, lasting several months, has established LHRH agonists as the dominant treatment in hormone therapy for prostate cancer.
- Initially co-administered with an androgen-receptor antagonist to block the LH and testosterone surge
- Initial exposure to LHRH agonists results in a surge of LH (up to 10x) and testosterone levels. This surge can result in a severe, life-threatening exacerbation of symptoms
- Co-administration of an anti-androgen (bicalutamide 50mg daily) functionally blocks the increased levels of testosterone.
- The testosterone flare may last for 10-20 days and therefore co-administration of anti-androgen is required for only 21-28 days
- Although some have argued that the administration of the anti-androgen should precede the administration of the LHRH agonist by at least a week, others have found no differences in PSA levels with the simultaneous administration of both agents.
- The testosterone flare may last for 10-20 days and therefore co-administration of anti-androgen is required for only 21-28 days
- Following the LH surge, the loss of phasic pituitary stimulation results in plummeting LH levels
- In the absence of LH, Leydig cell production of testosterone drops to castrate levels
- Dosing
- Goserelin (Zoladex) 10.8mg SC q3 months
- Leuprolide (Lupron) 22.5mg SC q3months
- LHRH antagonists (-lix (Abarelix, Cetrorelix, Degarelix, Relogolix))
- Advantages of LHRH antagonists over agonists:
- Does not require co-administration of an anti-androgen due to lack of LH surge from lack of agonist activity
- Testosterone levels can drop very quickly (within 3 days)
- May be preferred in hormonally naive patients in whom urgent castration is needed (impending spinal cord compression or severe bone pain) or in whom surgical castration is not appropriate
- LHRH antagonists bind immediately and competitively to the LHRH receptors in the pituitary, reducing LH concentrations by 84% within 24 hours of administration and testosterone levels dropping quickly with 34.5%, 60.5%, and 98.1% of men chemically castrate at 2, 4, and 28 days, respectively. [With LHRH agonists, the LH surge can last up to 2 weeks so testosterone levels only decrease after that§]
- In a phase III study, degarelix was compared to leuprolide: at 1 year of treatment degarelix was not inferior to leuprolide (Klotz et al, 2008)
- HERO
- Population: 930 patients with 1 of 3 clinical disease presentations:
- Evidence of biochemical (PSA) or clinical relapse after local primary intervention with curative intent
- Newly diagnosed hormone-sensitive metastatic disease
- Advanced localized disease unlikely to be cured by local primary intervention with curative intent.
- Randomized to in a 2:1 ratio, to receive relugolix (120 mg orally once daily) vs. leuprolide (injections every 3 months) for 48 weeks.
- Primary outcome: sustained testosterone suppression to castrate levels (<50 ng per deciliter) through 48 weeks.
- Secondary outcomes: noninferiority with respect to the primary end point, castrate levels of testosterone on day 4, and profound castrate levels (<20 ng per deciliter) on day 15.
- Results:
- Testosterone suppression: regurolix superior and non-inferior to leuprolide (96.7% regurolix vs. 88.8% leuprolide at 48 weeks)
- Secondary outcomes all improved with regurolix
- Cardiovascular outcomes significantly improved with regurolix (HR 0.46)
- Shore, Neal D., et al."Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer." New England Journal of Medicine (2020).
- Population: 930 patients with 1 of 3 clinical disease presentations:
- Many of the first- and second-generation antagonists induced significant histamine-mediated side effects; these do not occur as often observed in third- and fourth-generation.
- Nevertheless, severe allergic reactions can occur with abarelix, even after previously uneventful treatment
- Unlike abarelix, the LHRH antagonist degarelix has no systemic allergic reaction
- LH/FSH levels by method of ADT
- LHRH agonists: reduced LH and only partially suppressed FSH
- LHRH antagonists: reduce both LH and FSH levels
- Surgical castration: significantly elevated LH and FSH
- Inhibition of Androgen Synthesis
- Aminoglutethimide (historical)
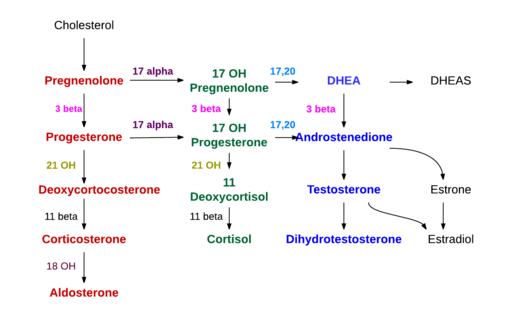
- Inhibits the conversion of cholesterol to pregnenolone, an early step in steroidogenesis.
- Given its inhibition of a very proximal step in adrenal function, aminoglutethimide blocks production of aldosterone and cortisol.
- As the medical version of a total adrenalectomy, the use of this agent requires replacement of cortisone and fludrocortisone.
- Ketoconazole (historical)
- Orally active, broad-spectrum antifungal agent
- Non-specific inhibitor of several cytochrome P450–dependent pathways, resulting in loss of adrenal steroid synthesis and testosterone synthesis by Leydig cells
- Effects are rapid, with testosterone levels dropping to the castrate level with 4 hours of administration in some cases. The effects are also immediately reversible, indicating dosing must be continuous (every 8 hours) to maintain low testosterone levels.
- Previously used as the first or second agent in so-called secondary hormonal manipulation for CRPC
- Adverse events include gynecomastia (caused by alterations in testosterone/estradiol ratios), lethargy, weakness, hepatic dysfunction, visual disturbance, and nausea.
- Because of the adrenal suppression, usually given with hydrocortisone (20 mg BID).
- Abiraterone acetate
- Orally active
- Potent, selective, non-steroidal, irreversible inhibitor of cytochrome P450 isoform 17 (CYP17)
- CYP17 has both 17,20-lyase and 17α-hydroxylase activity and is a key enzyme in androgen synthesis
- Inhibition of 17α-hydroxylase results in excess synthesis of aldosterone and its precursors, causing a suppression of cortisol with a compensatory rise in ACTH.
Steroidogenesis
Source: Wikipedia
- Blocks androgen synthesis by testis, adrenals, and prostate cancer cells that generate their own testosterone as part of a back door pathway
- More potent than ketoconazole
- Adverse events (7)
- HTN
- Hypokalemia
- Fluid overload
- Fatigue
- Hepatotoxicity
- Myopathy and rhabdomyolysis
- Increased triglycerides and cholesterol
- Generally well tolerated
- Hypertension, hypokalemia, and fluid overload
- Related to an increase in the mineralocorticoids, due to the effects of blocking the conversion of pregnenolone to 17-hydroxypregnenolone, resulting in an increase of the mineralocorticoids deoxycorticosterone and corticosterone.
- Abiraterone is co-administred with prednisone to suppress the increases in ACTH resulting from increased mineralocorticoids and decreased cortisol
- Concomitant steroid may exacerbate hyperglycemia in diabetics
- Hepatotoxicity
- Most serious potential adverse event and can be severe and potentially life threatening.
- Adverse event most likely to cause dose reduction or discontinuation.
- Liver enzymes as well as electrolytes must be checked frequently when initiating the medication.
- Recommended clinical monitoring (as per Cancer Care Ontario)
- Monitor for adrenal insufficiency: as clinically indicated when prednisone is withdrawn, or during periods of infection/stress
- Monitor for mineralocorticoid excess: as clinically indicated if patient continues on abiraterone after stopping prednisone
- Clinical assessment of adverse events: at each visit
- Blood pressure and serum potassium: baseline and monthly
- Liver function tests, bilirubin: baseline, every 2 weeks for the first 3 months and monthly thereafter, or as clinically indicated
- Cholesterol and triglycerides: baseline, every 2 to 3 months and as clinically indicated
- Trials with abiraterone
- COU-AA-301: post-docetaxel CRPC
- COU-AA-302: pre-docetaxel CRPC
- LATITUDE
- See below section on hSPC
- STAMPEDE
- Androgen-receptor antagonists i.e. anti-androgens
- Classified: steroidal vs. non-steroidal
- Steroidal anti-androgens (cyproterone acetate)
- Block androgen action at the cellular level
- Stimulates the AR in the hypothalamic-pituitary axis thereby activating negative feedback inhibition of the hypothalamus, resulting in decreased LH and testosterone§
- The classic steroidal antiandrogen cyproterone acetate rapidly lowers testosterone levels to 70-80%
- Adverse events (3):
- Fluid retention
- Thromboembolism
- Hypogonadism
- Non-steroidal anti-androgens (-lutamide)
- Block ARs, both at target tissues and in the hypothalamic-pituitary axis
- Inhibits the AR in the hypothalamic-pituitary axis thereby blocking negative feedback inhibition of the hypothalamus, resulting in increased LH and testosterone§
- Advantages over steroidal:
- Reduced risk of hypogonadism and sexual dysfunction (no anti-gonadotropic effects)
- With non-steroidal anti-androgens, testosterone levels reach about 1.5x the normal levels of hormonally intact men (recall steroidal anti-androgens reduce LH and testosterone)
- Potency may be preserved
- However, in clinical trials examining erectile functioning and sexual activity in men on flutamide monotherapy, long-term preservation of those domains was only 20%, not very different than men undergoing surgical castration.
- Reduced risk of osteoporosis
- Reduced risk of hypogonadism and sexual dysfunction (no anti-gonadotropic effects)
- Disadvantage over steroidal:
- Increased risk for adverse cardiovascular effects
- Adverse events:
- Liver toxicity
- Ranges from reversible hepatitis to fulminant hepatic failure
- Requires periodic monitoring of liver function tests
- Associated with all non-steroidal anti-androgens
- Gastrointestinal toxicity
- Most notably diarrhea
- More common with flutamide than the other non-steroidal anti-androgens
- Gynecomastia and breast pain
- Liver toxicity
- Due to the peripheral aromatization of increased testosterone to estradiol
- First-generation (flutamide, bicalutamide, nilutamide)
- MOA: acts by binding to the AR in a competitive fashion
- Flutamide
- Short half-life, three times per day dosing
- Bicalutamide
- Long half-life, once per day dosing
- Pharmacokinetics are not affected by age, renal insufficiency, or moderate hepatic impairment
- Associated with maintenance of serum testosterone levels in the majority of patients
- 150 mg/day bicalutamide monotherapy appears to have equivalent efficacy to medical or surgical castration in men with metastatic or locally advanced disease.
- Bicalutamide monotherapy (150 mg/day) is also associated with significantly better quality of life in the domains of sexual interest and physical capacity compared to surgical castration. There are, however, higher rates of gynecomastia (66.2%) and breast pain (72.8%)
- Nilutamide
- About 1/4 men on nilutamide therapy will note a delayed adaptation to darkness after exposure to bright illumination
- Second-generation: enzalutamide, apalutamide, darolutamide
- Enzalutamide
- MOA: irreversibly binds directly to the androgen receptor and inhibits the binding of androgens, AR nuclear translocation, and AR–mediated DNA binding
- Adverse events (note that first 6 also apply to apalutamide:
- HTN
- Diarrhea
- Fatigue
- Seizures
- <1% of patients in clinical trials
- Falls
- Fracture
- Hot flashes
- Neutropenia
- Memory impairment
- Arthralgia
- Recommended clinical monitoring (as per Cancer Care Ontario (accessed March 2020))
- Blood pressure: baseline and each visit
- ECG and electrolytes: Baseline and at each visit, in patients at risk of QT prolongation
- INR monitoring for patients on warfarin: baseline and at each visit
- Clinical assessment of adverse events: at each visit
- Trials with enzalutamide
- Apalutamide
- MOA: binds directly to the ligand-binding domain of the androgen receptor and prevents androgen-receptor translocation, DNA binding, and androgen-receptor–mediated transcription
- Adverse events (first 6 same as enzalutamide):
- HTN
- Diarrhea
- Fatigue
- Seizures
- <1% of patients in clinical trials
- Falls
- Fracture
- Hypothyroidism
- Rash
- Increased cholesterol
- Anemia
- Hyperglycemia
- Nausea
- Weight loss
- Arthralgia
- Recommended monitoring (as per Cancer Care Ontario)
- TSH: baseline and as clinically indicated
- ECG: baseline and as clinically indicated; more frequent in patients at risk of QTc prolongation
- INR: if warfarin cannot be discontinued; baseline and during apalutamide treatment
- Clinical assessment of adverse events: at each visit
- Trials with apalutamide
- Darolutamide
- Low penetration of the blood–brain barrier and low binding affinity for γ-aminobutyric acid type A receptors
- Advantage:
- Fewer and less severe toxic effects than apalutamide and enzalutamide
- Trials with darolutamide
- ARAMIS: M0 CRPC
Response to androgen blockage
- Following the initiation of ADT, the vast majority of prostate cancer patients will show some evidence of clinical response
- PSA response to ADT is associated with clinical outcomes; the magnitude and rapidity of that response remain the best predictors of its durability
- Assuming ADT effectively targets the androgen-sensitive population of prostate cancer cells, an incomplete or sluggish response is evidence of a significant androgen-refractory cell population
Combination therapy
- ADT with Radical Prostatectomy
- ADT with Radiation Therapy
- Combined androgen blockade (CAB)
- The term CAB is preferred over “total androgen blockade” or “maximum androgen blockade” since it is unknown what defines "total" or "maximum" blockade
- The concept of combining an anti-androgen to surgical or medical (LHRH analogue) castration is based on the idea that, after the elimination of testicular androgens through surgical or medical castration, adrenal androgens still contribute to prostate cancer progression
- The anti-androgens are nonspecific in blocking the binding of androgens to the AR: both testicular and adrenal androgens are affected.
- In a meta-analysis of 27 studies comparing CAB to standard ADT, there was no significant difference in 5-year survival (CAB 25.4% vs. 23.6% standard ADT).
- Studies including the steroidal antiandrogen cyproterone acetate had a slightly worse outcome on the CAB arms (5-year survival 15.4% vs. 18.1% for ADT alone), suggesting increased non–prostate cancer deaths in those receiving cyproterone acetate.
- When studies examining the outcomes of the non-steroidal antiandrogens flutamide or nilutamide were considered independent of those with cyproterone acetate, the 5-year survival improved significantly in the CAB arms by 3% (95% CI 0-5%) (27.6% with CAB vs. 24.7% for ADT)
Timing of therapy
- The timing of initiating ADT in non-metastatic disease remains one of the most controversial areas of prostate cancer management.
- The spectrum of opinions ranges from primary ADT at time of diagnosis to the initiation of ADT at the first sign of primary prostate cancer failure (early) to initiation of ADT only with objective evidence of distant metastatic disease (late)
- Early ADT in non-metastatic prostate cancer delays biochemical and clinical disease progression; the effect of early ADT on survival remains unclear
- ADT is indicated in symptomatic, metastatic disease
- Continuous ADT: immediate vs. delayed
- When considering immediate vs. delayed ADT, a few aspects about prostate cancer should be noted:
- The natural history of prostate cancer progression, even in the hormonally intact individual, is protracted, as demonstrated by the Pound et al. study. Therefore, even in the absence of ADT, men with progressive prostate cancer live for a long time
- Despite dramatic clinical responses, patients undergoing ADT either will die of a non–prostate cancer cause or will eventually demonstrate evidence of castration-resistant disease and die of prostate cancer
- ADT is associated with various side effects
- Immediate versus Delayed Androgen Deprivation Therapy: Integrating the Data
- In low-risk, localized prostate cancer, there is no survival benefit to primary, immediate ADT in low-risk, localized prostate cancer
- In fact, men treated with primary ADT have significantly worse OS than those spared ADT in this setting.
- In locally advanced, asymptomatic metastatic, the role of ADT is controversial
- In clinically present but undefined prostate cancer treated in a community setting with limited disease monitoring, immediate ADT results in significantly better prostate cancer–specific survival but not better overall survival.
- On the other hand, in patients deemed not suitable for local treatment, immediate ADT improved overall survival but not prostate cancer–specific survival.
- In N+ disease,
- Without primary treatment, there is no significant advantage to immediate ADT
- After radical prostatectomy, there is a significant survival advantage favoring immediate ADT, with a 2.6-year difference in median overall survival (ECOG 3886 Messing et al.).
Intermittent versus Continuous Androgen Deprivation Therapy
- In animal models, exposure to androgen deprivation on an intermittent—rather than continuous—basis lengthened the time to the emergence of androgen-refractory cancer growth
- In theory, the quality-of-life side effects of ADT on intermittent treatment should be improved compared to when ADT is used continuously.
- PR7
- Population: Patients with a rising PSA after primary or salvage radiotherapy received an 8-month induction of ADT and were then
- Randomized to intermittent vs. continuous ADT
- Intermittent treatment was provided in 8-month cycles, with nontreatment periods based on PSA levels.
- Primary outcome: OS
- Results:
- Median follow-up: 6.9 years
- OS non-inferior with intermittent ADT (8.8 years intermittent ADT vs. 9.1 years continuous ADT)
- Attrition from intermittent ADT progressively increased over time as patients either developed CRPC or died of another cause. Attrition occurred in 5% of men in the 1st interval, whereas 68% had stopped intermittent therapy by the 3rd interval.
- Duration of intermittent ADT progressively shortened over time
- Median nontreatment interval between cycles was 20.1 months for the 1st treatment cycle, 13.2 months for the 2nd cycle, 9.1 months for the 3rd, and 4 to 5 months thereafter.
- A secondary end point, improved quality of life in the intermittent therapy arm, was associated with significantly better scores for hot flashes, desire for sexual activity, and urinary symptoms. For the functional domains of physical, role, and global health the intermittent therapy arm was slightly better, but the differences were not significant. Overall, the authors concluded that “the difference in quality of life is not as profound as one might expect”
- Crook, Juanita M., et al. "Intermittent androgen suppression for rising PSA level after radiotherapy." New England Journal of Medicine 367.10 (2012): 895-903.
- Noninferiority trials require fewer subjects than an equivalence trial, making them easier to accrue and complete. It is important to recognize that noninferiority is not the same as equivalence; trial design is based on a definition of noninferiority if a prespecified upper margin of a hazard ratio is not exceeded. In this trial, the upper limit was 1.25, meaning up to 25% more men on the intermittent arm could die of any cause and intermittent treatment would still be considered noninferior. In this trial, the upper limit of the 95% CI was 1.22 (p=0.009), which is below 1.25 and therefore met the prespecified definition.
- SWOG 9346
- Population: patients with newly diagnosed metastatic prostate cancer whose PSA level had declined to ≤ 4 ng/mL after a 7-month induction of ADT (indicating androgen sensitivity)
- Randomized to intermittent vs. continuous ADT
- Co-primary outcomes: OS and QOL at 3 months after randomization
- Results
- Median follow-up: 9.8 years
- Median OS was significantly lower with intermittent ADT (5.8 years continuous vs. 5.1 years intermittent (HR for death with intermittent therapy, 1.10; 90% CI 0.99-1.23)).
- Unfortunately, these findings are statistically inconclusive: the CI for survival exceeded the upper boundary for noninferiority (1.20), therefore cannot conclude that intermittent therapy was noninferior to continuous therapy. Furthermore, because the lower limit of the CI (0.99) did not exclude 1.00, cannot conclude that intermittent therapy was significantly inferior to continuous therapy. A reasonable clinical interpretation of this statistically inconclusive study is that intermittent therapy is not superior to continuous therapy in men presenting with metastatic prostate cancer, and may be worse. In the words of the authors “given that nearly the entire confidence interval tends to favor continuous therapy, the results suggest that intermittent therapy may compromise survival”
- Hussain, Maha, et al."Intermittent versus continuous androgen deprivation in prostate cancer." New England Journal of Medicine 368.14 (2013): 1314-1325.
- Comparing the intermittent ADT trials
- Similarities
- Used an induction period of ADT (8 months and 7 months, respectively)
- Stopped ADT if PSA < 4 ng/mL
- CRPC was managed with continuous ADT
- Differences
- Patient population (post-radiation vs. newly diagnosed metastatic)
- PSA threshold to restart PSA (10 after radiation therapy or 20 ng/mL for metastatic disease)
- The treatment schedules provide some guidance but there is no consensus on the ideal schedule in managing patients on intermittent ADT
- Similarities
Economic considerations
- The cost of LHRH agonists ranged from greater than 10.7-13.5x times the cost of bilateral orchiectomy.
- The 3-month formulations of leuprolide acetate and goserelin become more expensive than orchiectomy upon the administration of a second 3-month depot
- Although DES is associated with increased cardiovascular toxicity, from a strictly cost point of view, it is the cheapest form of ADT
Complications of Androgen Ablation (16)
- COACH Wants BDSM From Montreal (16):
- Cardiovascular disease
- Osteoporosis
- Anemia
- Cognitive dysfunction
- Hot Flashes
- Weight gain and fat % mass increase
- Breast events
- Diabetes
- Sexual dysfunction
- Muscle % body mass decrease
- Fatigue
- Metabolic (5):
- Insulin resistance
- Glucose intolerance
- Increased triglycerides and total cholesterol levels
- Worsened glycemic control in men with a pre-existing diagnosis
- Increased risk of metabolic syndrome
- Cardiovascular disease
- Conflicting findings between observational studies and secondary analyses of randomized trials; overall, sufficient evidence to suggest a link between use of ADT and CVD
- Cardiovascular mortality§
- Meta-analyses of observational studies found significant increased risk
- Observational studies limited by confounding
- Meta-analyses of randomized trials found no significant association
- Secondary analyses of randomized trials limited by power
- Meta-analyses of observational studies found significant increased risk
- Nonfatal cardiovascular disease§
- Meta-analyses of observational studies found significant increased risk
- Meta-analyses of randomized trials found significant increased risk
- Myocardial infarction§
- 1 meta-analysis of observational studies found significant increased risk, 1 found no significant association
- Meta-analysis of randomized trials found no significant association
- Stroke§
- Some meta-analyses of observational studies found significant increased risk, others did not
- Meta-analysis of randomized trials found no significant association
- Venous thromboembolism
- 2021 CUA Guidelines on ADT Adverse Events: Insufficient evidence to recommend routine use of venous thromboembolism prophylaxis in men receiving ADT.
- Cardiovascular mortality§
- Pre-existing heart disease is significant risk factor for development of major adverse cardiac events (MACE) in men receiving
ADT.
- MACE is defined as myocardial infarction, coronary revascularization, stroke, and hospitalization because of heart failure.
- Nanda et al. JAMA 2009
- Population: 5077 males with localized or locally advanced prostate cancer treated with or without neoadjuvant ADT followed by RT
- Median duration ADT 4 months
- Primary outcome: all-cause mortality
- Results:
- No significantly increased risk of all-cause mortality in males with no comorbidity or a single coronary artery disease risk factor
- Significantly increased risk of all-cause mortality in males with coronary artery disease–induced congestive heart failure or myocardial infarction
- Nanda, Akash, et al."Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease–induced congestive heart failure or myocardial infarction." Jama 302.8 (2009): 866-873.
- Population: 5077 males with localized or locally advanced prostate cancer treated with or without neoadjuvant ADT followed by RT
- GnRH agonist vs. antagonist
- Animal studies suggest that GnRH agonists, but not antagonists, may induce plaque instability and rupture
- Meta-analysis of randomized trials found that GnRH antagonists were associated with lower risk of cardiovascular events than GnRH agonists (HR 0.44 (95% CI 0.26 – 0.74)§
- HERO trial (see above) found that MACE occurs in 3% of patients randomized to relugolix compared to 6% of patients randomized to leuprolide§
- 2021 CUA Guidelines on ADT Adverse Events: in males with a prior history of MI or stroke, consider use of a gonadotropin-releasing hormone (GnRH) antagonist
- Enzalutamide§
- Meta-analysis of observational studies found no significant increased risk of cardiac events
- Meta-analysis of randomized trials found no significant increased risk of cardiac events
- Significantly increased risk of hypertension
- Abiraterone§
- Meta-analysis of randomized trials found significant increased risk of cardiac events
- Pharmacovigilance study found significant increased risk of atrial tachyarrhythmia and heat failure§
- Trials underway to address cardiac outcomes in men receiving ADT
- PRONOUNCE
- Population: males with advanced prostate cancer
- Randomized to degarelix (GnRH antagonist) vs. leuprolide (GnRH agonist)
- Primary outcome: time from randomization to MACE
- RADICAL-PC: RAndomizeD Intervention for Cardiovascular and Lifestyle Risk Factors in Prostate Cancer
- Population: males with a new diagnosis of prostate cancer or recently initiated/about to initiate ADT
- Randomized to systematic cardiovascular and lifestyle risk factor modification strategy vs. usual care
- Primary outcome: time from randomization to MACE
- PRONOUNCE
- Osteoporosis
- Prostate cancer occurs mostly in older men, who are at increased risk for osteoporosis, even in the absence of ADT
- ADT associated with (3):
- Decreased bone mineral density (BMD)
- BMD loss occurs at a maximum rate during the first year of therapy, however, continues to decline with prolonged use of ADT
- Osteoporosis
- Osteoporosis is defined as BMD of 2.5 or more standard deviations below the peak bone mass for young adults (i.e., T-score ≤-2.5).
- Osteopenia (low bone mass) is defined as BMD more than 1.0 but less than 2.5 standard deviations below the peak bone mass for young adults (i.e., T-score <-1 and >-2.5).
- 4 years of ADT will place the average man in the osteopenia (precursor of osteoporosis) range.
- Increased risk for clinical fractures
- Shahinian et al. NEJM 2005
- Population: 50,613 males from SEER with a diagnosis of prostate cancer
- Comparison: patients treated with or without ADT
- Primary outcomes: occurrence of any fracture and pccurrence of a fracture resulting in hospitalization
- Results:
- Risk of facture within 5 years of prostate cancer diagnosis: 19% with ADT vs. 13% without ADT
- Risk of facture requiring hospitalization within 5 years of prostate cancer diagnosis: 5.2% with ADT vs. 2.4% without ADT
- Risk of fracture increased with increasing number of ADT doses
-
Shahinian, Vahakn B., et al. "Risk of fracture after androgen deprivation for prostate cancer." New England Journal of Medicine 352.2 (2005): 154-164.
- Shahinian et al. NEJM 2005
- Decreased bone mineral density (BMD)
- Anemia
- Very common
- Thought to be secondary to lack of testosterone stimulation of erythroid precursors and a decrease in erythropoietin production
- Usually normochromic, normocytic
- Hemoglobin levels decrease by 1–2 ng/dL from baseline
- 90% of men receiving combined androgen blockade experienced declines in hemoglobin concentration of at least 10%
- Cognitive dysfunction
- ADT in men with PCa may be associated with (3):
- Changes in cognition (concentration, memory)
- Depression
- Dementia
- Evidence related to causality remains weak and further prospective data are needed
- Changes in cognition
- Conflicting findings
- Some studies find reduced cognition
- Self-reported changes in concentration, information processing, verbal fluency, visual information processing, visuospatial function, memory, and executive function, neuro-fatigue and apathy
- Objective changes in verbal memory, spatial abilities and attention
- Some studies find no change in cognition
- One study reported improvement in episodic memory and delayed recall§
- Conflicting findings
- Depression
- Meta-analysis of 6 studies comprising 226,871 patients found significantly increased risk (HR 1.51 (95% CI 1.34 – 1.69))§
- Dementia
- Meta-analysis of 9 studies comprising 442,665 patients found significantly increased risk (HR 1.21 (95% CI 1.11 – 1.33))§
- Alzheimer’s disease
- Meta-analysis of 8 studies comprising 1,597,546 patients found significantly increased risk (HR 1.16 (95% CI 1.09 – 1.24))§
- Hot flashes
- Common and bothersome side effect of ADT
- Among the most common adverse events associated with androgen ablation, affecting between 50-80% of patients
- If bothersome, they can lead to a deterioration in quality of life and may decrease compliance to ADT
- Described as a subjective feeling of warmth in the upper torso and head followed by objective perspiration.
- Generally decrease in both frequency and intensity over time but often persist in some men
- Weight gain and fat % mass increase
- Largely due to an accumulation of subcutaneous fat, rather than intraabdominal adipose tissue
- Thought to occur soon after initiating therapy, sometimes as early as 1 month following treatment
- Weight increases on average by 2.1% and % fat mass increases on average by 8%§
- Longer duration of therapy appears to increase weight gain and percentage fat mass
- Changes may persist up to 2 years beyond treatment cessation
- Breast events
- Breast events include gynecomastia (increased amount of breast tissue) and mastodynia (breast tenderness).
- May occur concurrently or separately
- Gynecomastia
- Occurs as a result of peripheral conversion of testosterone to estradiol, which increases the ratio of estrogen to androgen activity
- Occurs most commonly with androgen-receptor antagonist monotherapy
- Rare complication of LHRH monotherapy or combined androgen blockade
- Diabetes
- Alibhai et al. JCO 2009
- Population: matched cohort study 38,158 males aged 66 or older with prostate cancer
- Comparison: males given continuous ADT for at least 6 months or underwent bilateral orchiectomy vs. those without
- Primary outcomes: acute myocardial infarction, sudden cardiac death, diabetes
- Results:
- Significantly increased risk of incident diabetes (hazard ratio 1.16 (95% CI 1.11 - 1.21))
- Alibhai, Shabbir MH, et al."Impact of androgen deprivation therapy on cardiovascular disease and diabetes." Journal of clinical oncology: official journal of the American Society of Clinical Oncology 27.21 (2009): 3452.
- Sexual dysfunction
- Impacts multiple domains of sexual function, including
- Loss of libido (in up to 90% of men)
- Erectile dysfunction
- Decreased sensitivity to sexual stimulation
- Decreased penile and testicular size
- Body image
- A study of 39 males with non-metastatic prostate cancer initiating ADT found that stretched penile length decreased from an average of 10.76 cm to 8.05 cm after 15 months of ADT and plateaued thereafter.§
- Only up to 20% of men on ADT are able to maintain some sexual activity. Libido is more severely compromised, with ≈5% of men maintaining a high level of sexual interest with ADT
- Muscle mass % decrease
- A decrease in muscle mass causes a decrease in grip strength, absolute muscular strength, and gait speed.
- ADT also results in detrimental changes to multiple other physical parameters, including aerobic fitness and overall physical function
- Noticeable side effect of ADT
- Underlying cause is often multifactorial
- Metabolic (5):
- Insulin resistance
- Glucose intolerance
- Worsened glycemic control in men with a pre-existing diabetes
- Increased triglycerides, low-density lipoprotein (LDL), and total cholesterol levels
- Increased risk of metabolic syndrome
- Braga-Basaria et al. JCO 2006
- Population: Cross-sectional study of 58 males from 3 groups:
- PCa who were undergoing ADT (for recurrent or metastatic disease) for at least 12 months before the onset of the study and were in clinical and biochemical remission (ADT group)
- Age-matched men with nonmetastatic PCa who had undergone prostatectomy and/or radiotherapy and were recently found to have an increasing prostate-specific antigen (PSA) level but had not received ADT and were eugonadal (non-ADT group)
- Age-matched healthy eugonadal men with a normal PSA (control group)
- Results
- ADT group had significantly higher body mass index, prevelanece of metabolic syndrome, abdominal obesity, and hyperglycemia
- ADT group had significantly lower total and free testosterone levels.
-
Braga-Basaria, Milena, et al."Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy." Journal of clinical oncology 24.24 (2006): 3979-3983.
Recommended Investigations Prior to ADT initiation (2021 CUA Guidelines§)
- History and Physical
exam (4)
- History (4)
- Cardiometabolic
- History of major adverse cardiac events (MACE)
- Risk factors for cardiac disease
- Previous VTE or stroke
- Bone
- Falls risk
- Cardiometabolic
- Physical exam (5)
- Cardiometabolic
- Blood pressure
- Weight
- Waist circumference
- Calculation of body mass index (BMI)
- Bone
- Height
- Cardiometabolic
- History (4)
- Labs
(7)
- Cardiometabolic
- Diabetes screening (fasting plasma glucose, oral glucose tolerance test, or Hgb A1c level)
- Lipid profile:
- Triglycerides
- Low-density lipoprotein [LDL] cholesterol
- High-density lipoprotein [HDL] cholesterol
- Total cholesterol
- Bone
- Calcium
- 25-hydroxyvitamin D
- Cardiometabolic
- Other (2)
- Cardiometabolic
- Referral to a cardiologist or cardio-oncologist may be considered in patients with a history of myocardial infarction (MI) or stroke, for assessment and medical optimization prior to initiating ADT
- Bone
- Bone mineral density (BMD) testing using dual energy x-ray absorptiometry (DXA)
- Using a validated tool, results of BMD testing
should be used to
calculate a patient’s 10-year risk of a major osteoporotic
fracture for consideration of pharmacological therapy.
- Recommended tools for calculating fracture risk
- Using a validated tool, results of BMD testing
should be used to
calculate a patient’s 10-year risk of a major osteoporotic
fracture for consideration of pharmacological therapy.
- Bone mineral density (BMD) testing using dual energy x-ray absorptiometry (DXA)
- Cardiometabolic
- Inform primary care provider that the patient has been initiated on ADT and that there may be adverse events associated with this therapy
- Counsel patient regarding sexual side effects, particularly with respect to body image.
Prevention & Management of Complications on ADT (2021 CUA Guidelines§)
- Cardiometabolic
- Lifestyle changes (smoking cessation, dietary
modifications, exercise)
- Patients should be encouraged to attend supervised
exercise programs using a combination of resistance
and aerobic training
- Supervised exercise therapy in men with PCa is superior to self-implemented exercise regimens
- Benefits of exercise in males on ADT (10)
- Physical domains (5):
- Prevention of muscle loss and resultant decline in lean body mass
- Decreased body mass index
- Improved muscle strength
- Improvements in peak oxygen consumption and endothelial function
- Improved overall physical function
- Functional domains (2):
- Lower levels of fatigue
- Decreased risk of falls and fractures
- Lower levels of fatigue
- Endocrine domains (2):
- Improved insulin and glucose homeostasis
- Improved in lipid profile
- Improved insulin and glucose homeostasis
- Multiple health-related quality of life domains
- Physical domains (5):
- Patients should be encouraged to attend supervised
exercise programs using a combination of resistance
and aerobic training
- Monitor blood pressure and treat hypertension for a target of <130/80
- Diabetes screening and lipid profile (see above) assessments should be continued
at 6–12 month-intervals throughout treatment duration
- Dyslipidemia should be treated according to current best practice guidelines
- Dyslipidemia should be treated according to current best practice guidelines
- Lifestyle changes (smoking cessation, dietary
modifications, exercise)
- Bone health
- Lifestyle changes
- Smoking and alcohol cessation
- Smoking and alcohol
use are associated with bone loss and fractures
- Smoking and alcohol
use are associated with bone loss and fractures
- Exercise therapy using a combination of resistance and aerobic training, preferably in a supervised setting
- Exercise may preserve BMD in men receiving ADT
- Smoking and alcohol cessation
- Calcium intake
(1200 mg daily total from diet and supplements) and vitamin
D supplementation (800–2000 IU daily)
- No evidence that shows this decreases risk of BMD loss or fractures in men receiving ADT but have been shown to prevent fractures in the general population age > 50
- Pharmacotherapy
- See Bone Health section in Castrate-Resistant Prostate Cancer Chapter Notes
- Bisphosphonates (zoledronic acid, alendronate, and pamidronate) can increase bone mineral density in men on ADT
- Indications for pharmacotherapy (4):
- Osteoporosis
- History of fragility fractures in the hip or spine
- History of multiple fragility fractures
- Moderate or high 10-year fracture risk
- Other guideline-based indications for pharmacotherapy
- 2019 CUA CRPC Guidelines recommend denosumab or zoledronic acid every 4 weeks, along with daily calcium and vitamin D in men with CRPC and bone metastases
- 2020 CUA CSPC Guidelines recommend that all men treated with ADT require vitamin D supplementation (800-1200IU daily) and calcium supplementation (800mg-1000mg total intake daily). Those at high risk of fractures should be treated with bone targeted therapy (zoledronic acid 5mg once a year, alendronate 70mg weekly, denosumab 60mg every 6 months).
- Surveillance DXA (until treatment
cessation)
- Every 2–3 years in low 10-year fracture risk
- Every 1-2 years in
- Osteopenia
- Moderate or high risk for fractures
- Patients started on pharmacological therapy should have followup DXA to assess for treatment response.
- Lifestyle changes
- Hot flashes
- Lifestyle changes
- Avoidance of potential patient-identified triggers, commonly heat or spicy foods
- Pharmacological (5) (none are Health Canada-approved for hot flashes):
- Medroxyprogesterone acetate (Provera) 20 mg orally daily
- Megestrol acetate (Megace) 20 mg orally twice daily
- Cyproterone acetate (Androcur) 50–100 mg orally daily
- Gabapentin (Neurontin) 900 mg orally daily
- Venlafaxine (Effexor) 75 mg orally daily
- Few case reports describe progression of prostate cancer with megestrol acetate; therefore, monitoring of disease is important
- The best pharmacological therapy to treat hot flashes remains unclear
- Randomized trial comparing medical therapy for hot flashes
- Population: 311 males with prostate cancer treated with ADT and experiencing hot flashes
- Randomized to venlafaxine, medroxyprogesterone acetate, or cyproterone acetate
- Primary outcome: change in median daily hot-flush score between randomization and 1 month
- Results:
- Decreases in hot-flush score were significantly larger in the cyproterone and medroxyprogesterone groups than in the venlafaxine group
- Irani, Jacques, et al."Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin-releasing hormone analogues for prostate cancer: a double-blind, randomised trial." The lancet oncology 11.2 (2010): 147-154.
- Randomized trial comparing medical therapy for hot flashes
- Intermittent ADT improves hot flashes and should be considered in appropriately selected patients
- Acupuncture may have a beneficial effect and can be considered in patients unwilling or unable to use pharmacotherapy.
- Lifestyle changes
- Breast events
- Options (3):
- Tamoxifen (selective estrogen receptor modulator)
- Effective for prophylaxis and treatment of breast events
- Radiation therapy (10 Gy)
- Effective for prophylaxis of both gynecomastia and mastodynia and treatment of mastodynia; radiation has no benefit once gynecomastia has begun
- Surgical (select patients with gynecomastia)
- Prophylaxis for the prevention of gynecomastia in men receiving ADT is not currently recommended
- Tamoxifen is more effective than a single 12-Gy fraction of RT for prophylaxis and treatment of breast events
- Tamoxifen (selective estrogen receptor modulator)
- Options (3):
- Cognitive function
- Monitor for cognitive decline and depression throughout duration of treatment
- Fatigue
- Best treated with exercise therapy
- Anemia
- Mild in most cases and often does not warrant treatment.
- Reversible after stopping ADT, but may take up to 1 year
- Indications for hematology referral (2):
- Severe anemia
- Decline in hemoglobin that exceeds the expected response to ADT alone
- Sexual function and body image
- Consider referral to a sex therapist in males desiring improved sexual function
- Erectile dysfunction may be treated with various interventions, including phosphodiesterase inhibitors; however, treatment efficacy may be poor without adequate mental and physical arousal
- Treatment for loss of libido in males on ADT is difficult
- Intermittent ADT may improve libido and erectile function and should be considered in appropriately selected patients
- Health related quality of life
- Exercise therapy should be encouraged to improve HRQOL during treatment
- Intermittent ADT improves HRQOL and should be considered in appropriately selected patients.
- In general, men with non-metastatic PCa are
likely to benefit from intermittent ADT without major concern
for compromised oncological outcomes, while those with metastatic PCa should be considered for intermittent therapy with caution.
- In general, men with non-metastatic PCa are
likely to benefit from intermittent ADT without major concern
Questions
- What are the current therapeutic approaches for androgen-axis blockade?
- How long does it take to achieve castration levels of testosterone following bilateral orchiectomy?
- What are the advantages of LHRH antagonists over agonists?
- What happens to LH and FSH levels after surgical castration? LHRH agonist? LHRH antagonist?
- Compare the hormonal and toxicity profile of a steroidal to non-steroidal anti-androgens.
- What are the potential adverse effects associated with the use of enzalutamide? Abiteraterone? Apalutamide?
- What are the potential adverse effects associated with the use of ADT?
Answers
- What are the current therapeutic approaches for androgen-axis blockade?
- Inhibit androgen synthesis
- Inhibition of androgen sources
- Inhibition of LHRH release
- Androgen-receptor blockade
- How long does it take to achieve castration levels of testosterone following bilateral orchiectomy?
- 24 hours
- What are the advantages of LHRH antagonists over agonists?
- No need for antiandrogen administration due to lack of LH surge with antagonists
- Testosterone levels drop quickly
- What happens to LH and FSH levels after surgical castration? LHRH agonist? LHRH antagonist?
- Surgical castration: elevated LH and FSH
- LHRH agonist: suppressed LH, partially supressed FSH
- LHRH antagonist: suppressed LH and FSH
- Compare the hormonal and toxicity profile of a steroidal to non-steroidal anti-androgens.
- Steroidal: fluid retention, thromboembolism, hypogonadism; decreased LH and testosterone
- Non-steroidal: liver toxicity, GI toxicity, gynecomastia and mastodynia; increased LH and testosterone
- What are the potential adverse effects associated with the use of enzalutamide? Abiteraterone? Apalutamide?
- Enzalutamide: hypertension, fatigue, diarrhea, seizures, falls, fracture, hot flashes
- Apalutamide: hypertension, fatigue, diarrhea, seizures, falls, fracture, hypothyroidism, rash, increased cholesterol, hyperglycemia, anemia
- Abiraterone: hypertension, fatigue, fluid retention, hypokalemia, hepatotoxicity, myopathy and rhabdomyolysis, increased triglycerides and cholesterol
- What are the potential adverse effects associated with the use of ADT?
- COACH Wants BDSM From Montreal
- Cardiovascular disease
- Osteoporosis
- Anemia
- Cognitive dysfunction
- Hot flashes
- Weight gain and % fat increase
- Breast events
- Diabetes
- Sexual dysfunction
- Muscle mass decrease
- Fatigue
- Metabolic
References
- Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds): CAMPBELL-WALSH UROLOGY, ed 11. Philadelphia, Elsevier, 2015, chap 120
- Kokorovic, Andrea, et al. "Canadian Urological Association guideline on androgen deprivation therapy: Adverse events and management strategies." Canadian Urological Association journal= Journal de l'Association des urologues du Canada 15.6 (2021): E307-E322.